Solar System/Extraterrestrial Water
This topic is incomplete. |
This page is about extraterrestrial water, the main Solar System topic for 2014 and 2015. To learn more about the Solar System general competition, please see the main Solar System page.
Extraterrestrial Water is a topic of the event Solar System. It covers several planets and moons in the solar system that are candidates for possible extraterrestrial water, or have the possibility of sustaining extraterrestrial life.
Extraterrestrial Water
During the 2014 and 2015 seasons, Solar System focused on extraterrestrial water within the solar system. This section only goes over the aspects of the celestial bodies that are associated with water.
Mars
Water on Mars is very rarely found as a liquid, as the pressure is too low at the surface for it to form. Water is mainly found as solid ice, but there is some gaseous water vapor in the thin atmosphere. Ancient Mars could have had a denser atmosphere, allowing liquid water to be present at the surface. Channels eroded by floods, ancient river valley networks, deltas, and lake beds all point to the idea of ancient liquid water. Water has been found in ice form at the bottom of some craters in the mid latitudes, most notably in the Vastitas Borealis crater with the Mars Express orbiter. In the southern Elysium Planitia, there is what appears to be plates of broken ice. The ice is speculated to have been formed from water that had spewed out of the fault Cerberus Fossae about 2 to 10 million years ago. The poles have water ice layers that vary in thickness from summer to winter. In the summer, the amount of water ice decreases in the poles as it sublimates into the atmosphere.
Discovery of Water
The Mars Express, using its MARSIS radar sounder, targeted the south ice cap and confirmed that ice is present at the cap in 2004. The OMEGA instrument indicated that the ice was separated into three parts; the top, reflective part, the slopes called scarps that fall away to the surrounding plains, and the permafrost that stretches for kilometers away from the cap. The Phoenix lander discovered the presence of water within its landing site near the north ice cap in July 2008. The Mars Reconnaissance Orbiter two years later found that the volume of ice in the north ice cap was 821,000 cubic kilometers.
Furthermore, patterned grounds characteristics of Earth's periglacial regions have been found on some Martian surfaces. A radar study in January 2009 looking at lobate debris aprons in Deuteronilus Mensae found evidence for ice lying beneath a few meters of rock.
Glaciers have been reported in numerous Martian craters. The Gamma Ray Spectrometer on Mars Odyssey and measurements on the surface from the Phoenix lander have pointed to the idea of ground water under Mars's surface. Areas of Mars in mid to high latitudes are thought to have large amounts of water ice. Recent evidence has shown that glaciers could be hidden under insulating rocks and/or dust.
Evidence found by the Mars Reconnaissance Orbiter have shown that sometime in the past ten years, a liquid had deposited sediment within a gully. This was found in the craters Terra Sirenum and Centauri Montes. In August 2011, a Nepalese student, Lujendra Ojha, found seasonal changes on slopes near crater rims in the Southern hemisphere. These streaks seemed to grow in the summer, and fade the rest of the year. It is thought that salty water (or brines) flow downhill and evaporate, leaving a mineral deposit. These slope lines are in sync with the heat flux of the Martian surface. The rate of growth with the features are consistent with groundwater flow through a sandy stratum.
Europa
Europa is thought to have more water on and under its surface than Earth. Europa has an outer layer of frozen water ice. Below that, it is theorized that there is a liquid saltwater ocean. Galileo orbiter found that the ocean creates a magnetic field out of Jupiter's. The surface ice floats on top of the ocean and drifts, much like the Earth's lithosphere on the asthenosphere.
Tidal heating exerted by Jupiter and the other Galilean moons is the leading explanation for Europa's liquid ocean. Jupiter's large gravitational force slightly stretches the moon and as the moon rotates around the planet, different parts of the moon stretch and compress. The other moons add to this as they pass by. This creates heat within the moon's interior and thus melts the lower layers of Europa's ice sheet. Water on Europa, solid or liquid, creates a layer about 100 km thick in total.
There are two models on how this water layer behaves, the "thick ice" theory and the "thin ice" theory. The thick ice theory states that there is an outer layer comprised of solid ice and plastic "warm ice" layer, and that the rest of the water layer is a liquid ocean. This theory shows that the ocean has rarely interacted with the surface. The thin ice theory states that Europa has a solid outer layer only a few kilometers thick. The model considers only the topmost layer that acts elastically under Jupiter's tides, and considers the interaction of Europa's surface and the ocean. According to the model, the ocean could interact with the surface through open ridges and form chaotic terrain.
Much like the moon of Enceladus, Europa has reoccurring plumes of water around 200 km high, which occur at Europa's aphelion and disappear at perihelion, due to the tidal force of Jupiter and its cycle.
Enceladus
Enceladus, much like Europa, is thought to have an ocean under surface ice. This ocean, unlike Europa's, is located near the South pole, and is thought to have around the same volume of water as Lake Superior. The ocean is thought to be caused by tidal heating from Saturn. It is also speculated that the area has other origins of heat, such as radioactive heating, sublimation of ice, shear heating, and that certain chemicals within the ocean (such as ammonia) lower the freezing point of water.
Enceladus's surface is made of water ice. It is fairly active with its relatively smooth surface, which is especially smooth around the southern tiger stripes. The tiger stripes are areas of high cryovolcanism, which involves the eruption of water and other volatiles that are not silicate rock. The geysers on Enceladus release mostly water vapor and some other components such as nitrogen, methane, and carbon dioxide. The materials are deposited around the geysers, covering most rugged terrain in the area. The geysers are thought to be created much like geysers here on Earth, and emit from pressurized water chambers that are heated from tidal heating or other heating methods listed above.
The eruptions are correlated with Enceladus's orbit and its distance from Saturn. When the moon is at aphelion, more material erupts from the geysers, and at perihelion, the geysers release less material. This is due to the tidal effects of Saturn which pull the tiger stripes open at aphelion and compress them at perihelion. These geysers are thought to be a factor in the formation of Saturn's E ring.
Enceladus has a thick atmosphere compared to the other moons of Saturn, besides Titan. The atmosphere could be formed from cryovolcanism or escaped particles from the surface or interior. The atmosphere consists of mostly water vapor (91%) and some nitrogen (4%), carbon dioxide (3.2%), and methane (1.7%).
Iapetus
Iapetus is thought to be composed mostly of ice, as it has a low density. The moon is known for its two tone coloration, that one side is darker than the other. The dark side is called Cassini Regio and the light side is separated into two parts: Roncevaux Terra in the north and Saragossa Terra in the south. This coloration is likely caused by sublimation of ice on the darker, warmer side, which produces vapor that deposits on the lighter, cooler side. As more ice sublimates on the dark side, darker material is shown and the temperature increases, which in turn kick-starts more sublimation and deposition.
This cycle occurs on both sides, but more intensely on Cassini Regio. It has been calculated that every billion years at current temperatures, Cassini Regio looses 20 meters of ice while the other side loses 10 centimeters. Ice does move from the light side to the dark side but at a slower rate.
The sublimation leaves lag (residue) on the dark side, giving the side its characteristic reddish color. The lag consists of organic materials much like on meteorites from the early solar system. It has been found that the lag creates a foot-deep layer above a layer of ice. The distribution of sublimation processes (described in the final sentences of the previous paragraphs) is the cause for the thinness of the layer. Cassini Regio also remains dark from the impact sunlight has on the lag; sunlight darkens the particles as they reside on the surface or travel in orbit around the moon.
Iapetus is also known for its equatorial ridge, which resides along the center of Cassini Regio. There is a theory that states that the ridge was formed from an upwelling of icy material below the surface that solidified. Other theories say it was formed from Iapetus's supposed early oblate shape, the deposition of a ring system around the moon, or a convective overturn.
Triton
Triton is the largest moon of Neptune and has a surface covered with various ices. Most of the surface is frozen nitrogen (55%) with water ice coming in at second (15-35%), and carbon dioxide making up the remaining 10-20%. Water comprises Triton's mantle, which is above a core of rock. Scientists believe that if Triton has a large enough core, radioactive decay and tidal heating could create enough heat for convection to occur in the mantle, or even the ocean proposed above, meaning that life could occur in the moon.
Water ice is what comprises the "cantaloupe" terrain on the western hemisphere of the moon. More specifically, the ice is dirty water ice, with frozen gases and dust mixed in. This cantaloupe terrain is the oldest terrain on the moon and consists of many depressions that are not impact craters due to their similar size and smoothness. Scientists theorize it could be caused by diapirism, the rising of lumps of less dense materials within more dense materials.
Other theories suggest that the terrain is caused by collapses or flooding from cryovolcanism. On Triton, nitrogen erupts into the atmosphere through the process of cryovolcanism. It is thought to be caused by the same method as most examples of this on other bodies in the solar system, through some sort of heat source. Tidal heating could heat nitrogen beneath the surface, making it expand and force its way up to the surface through vents. Another source could be a greenhouse effect created by solid materials on the moons icy surface. Solar radiation passes through the ice, heating nitrogen below and within it, and pressure from the nitrogen continues until it erupts.
Cryovolcanism on Triton does not release water, but water can be found in supposed icy lava on the surface. Cipango Planum on the eastern hemisphere is a high plain thought to be caused by the accumulation of icy lava, which is thought to be comprised of ammonia and water.
Ceres
Ceres is the largest object in the asteroid belt, being the only dwarf planet there. It is also one of the listed potential sites of extraterrestrial life, although it has not been considered as much as other bodies. Ceres's surface has been found to be composed of some hydrated minerals (minerals with water in their chemical structure), which is evidence for water in the interior.
Ceres has an oblate shape that is common with a differentiated body, which points to the idea that Ceres may consist of a rocky core with an icy mantle above. As found by the Keck Telescope in 2002, this mantle contains 200 million cubic kilometers of water, more than the amount of fresh water on Earth. Characteristics of its surface points to the possibility of volatile materials in the body. Some still speculate that Ceres could only be partially or not differentiated with a porous composition. This theory states that a rock layer on a mantle of ice would sink down and create salt deposits, something not found on Ceres. Theorists say that Ceres doesn't have an ice shell, but has water mixed throughout the body.
Ceres's atmosphere is thin but is comprised of water vapor, which is speculated to have been released by the sublimation of water ice that has migrated from the interior. Some evidence for this was found in the 1990s at Ceres's north pole but was never proven. The IUE spacecraft found hydroxide ions near the pole through ultraviolet observations, which are released when water vapor is split apart by solar radiation. It also has been found that there could be a water vapor source(s) at the mid-latitudes. The Herschel Space Observatory in early 2014 found that localized water sources in this area give off around 3 kilograms of water vapor a second. It is thought that this water is released from sublimation of surface ice or even cryovolcanism created by supposed radioactive energy. The Dawn spacecraft arrived at Ceres in 2015 and continues to help our understanding of the planet.
Titan
Titan is the largest moon in our solar system. Like Europa and Enceladus, it is thought to have a subsurface water ocean. Titan is thought to have a rocky center surrounded by a water layer that is thought to be differentiated. The lowest layer is comprised of high-pressure ice, such as Ice VI with tetrahedron crystals. The next layer is comprised of liquid water and the top layer is normal ice 1. The topmost layer of Titan is thought to be very rigid and varying in thickness, based on gravity field tests taken by Cassini. The gravity tests also show that the moon must have a high density, showing that the subsurface ocean most likely a dense, salty brine. The varying thickness could possibly be caused by an ocean that is slowly crystallizing.
The liquid layer is thought to be almost like a magma made of liquid water and ammonia. The ammonia is thought to make the water buoyant enough to bubble up through the icy crust, like magma on Earth. The ocean is also thought to have a high amount of dissolved salts made out of sulfur, sodium, and potassium. This makes the ocean almost like a brine and around as salty as the saltiest bodies of water on Earth (like the Dead Sea and the Great Salt Lake). Because of the characteristics of this ocean, it is likely that when it is forced through the crust, it takes methane from the ice. The ocean could also be a reservoir for methane. This could be a reason for the high amount of methane in the atmosphere and on the surface.
Evidence for this ocean comes from the Cassini probe. The probe detected extremely low-frequency radio waves in the atmosphere, whereas the surface of Titan is thought to be a poor reflector of low-frequency waves. The waves in the atmosphere are thought to have been reflected off of a liquid-ice boundary of a subsurface ocean. Cassini also found that the surface of Titan has solid tides up to 30 feet in height, which would not be possible for a body with a solid rocky composition. Because of this, scientists theorize that a liquid layer allows the tides to occur this high.
Researchers also speculate that Titan has cryovolcanism at its surface, which most likely would spew out the ammonia-water of Titan's supposed ocean. But because Titan's outer layer is comprised of ice 1, which is less dense than liquid water, there would have to be a large amount of energy powering cryovolcanism. There would have to be tidal heating from Saturn and radioactive decay for there to possibly be enough energy for this activity to work.
Pressure from underplating of ice plates at the surface could also drive cryovolcanism. Underplating occurs when one tectonic plate subducts under another and partially melts. This melting could create some plume events much like what happens at Earth's subduction zones. This theory could only be true if Titan has tectonic activity occurring at its surface, but data taken from the moon provides evidence for tectonic activity.
Comets
Comets consist of a nucleus (a solid, core structure), coma ("atmosphere" around the nucleus), and two tails (a gas tail and a dust tail). The nucleus consists of a conglomerate of rock, dust, water ice, and other frozen gases (carbon dioxide, carbon monoxide, methane, etc). The nucleus's water ice is hidden under a surface crust around several meters thick. This crust reflects little light as it is comprised mostly of organic compounds, which allows more heat to be absorbed. Solar heating drives off lighter volatiles, leaving heavy dark compounds much like tar or crude oil.
The solar heating enables the process of outgassing to occur. Outgassing is the process by which gases that are trapped, dissolved, frozen, or absorbed are released. Gases on a comet are released as jets off of the nucleus's surface, which are formed from the uneven heating of the nucleus's surface. These jets consist of water vapor and ice, carbon dioxide, and other trapped gases within the comet (listed above). The outgassing process creates a coma of water and dust from the comet's nucleus, which forms around the nucleus. As the comet travels closer to the sun, more water is released from the nucleus, increasing its amount in the coma.
Due to the amount of water in most comets, scientists have theorized that they contributed to the introduction of water to the Earth. However, tests on comets have shown evidence that says otherwise. Water on comets includes a higher amount of heavy hydrogen (hydrogen with a neutron with the proton) than Earth's water, around 300 ppm instead of 150 ppm on Earth. Even though some comets have water much like Earth's, most tested comets have the wrong amount. Comets also have been speculated to be what brought amino acids to Earth due to the high amount of organic chemicals in most comet's nuclei. Some even speculate comets brought organisms to early Earth. However, scientists believe that meteorites brought water and organic compounds to Earth. Specifically, meteorites called carbonaceous chondrites have water much like Earth's, leading to theories that say these brought water to Earth. Yet, comets are still not out of the picture as a potential source of water on Earth.
Properties of Water
This section requires cleanup to meet the wiki's quality standards. |
For the 2014-15 Solar System event, it is crucial to know the properties of water in all phases.
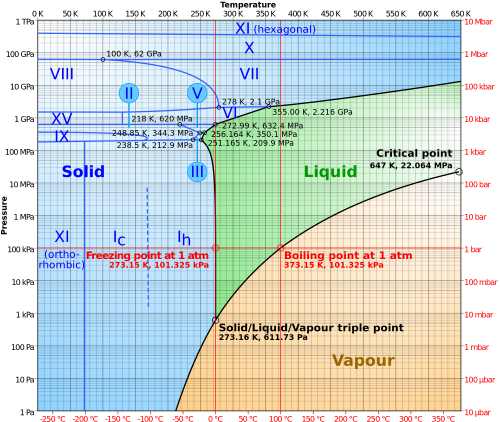
Phase Diagram
Water Ice Forms
This section is incomplete. |
Ice may be in an amorphous solid state at various densities or any one of the 17 known solid crystalline phases of water.
Subjected to high pressures and varying temperatures, ice can form in sixteen separate known phases. The types are differentiated by their crystalline structure, ordering and density. There are also two metastable phases of ice under pressure, both fully hydrogen-disordered; these are IV and XII. Ice XII was discovered in 1996. In 2006, XIII and XIV were discovered. Ices XI, XIII, and XIV are hydrogen-ordered forms of ices Ih, V, and XII respectively. In 2009, ice XV was found at extremely high pressures and −143 °C. At even higher pressures, ice is predicted to become a metal; this has been variously estimated to occur at 1.55 TPa or 5.62 TPa.
As well as crystalline forms, solid water can exist in amorphous states as amorphous ice (ASW) of varying densities. Water in the interstellar medium is dominated by amorphous ice, making it likely the most common form of water in the universe. Low-density ASW (LDA), also known as hyperquenched glassy water, may be responsible for noctilucent clouds on earth and is usually formed by deposition of water vapor in cold or vacuum conditions. High density ASW (HDA) is formed by compression of ordinary ice Ih or LDA at GPa pressures. Very-high density ASW (VHDA) is HDA slightly warmed to 160K under 1–2 GPa pressures.
In outer space, hexagonal crystalline ice (the predominant form found on Earth) is extremely rare. Amorphous ice is most common.
Low Density Amorphous
Low-density amorphous ice, also called LDA, vapor-deposited amorphous water ice, amorphous solid water (ASW) or hyperquenched glassy water (HGW), is usually formed in the laboratory by a slow accumulation of water vapor molecules (physical vapor deposition) onto a very smooth metal crystal surface under 120 K. In outer space it is expected to be formed in a similar manner on a variety of cold substrates, such as dust particles. It is expected to be common in the subsurface of exterior planets and comets.
Melting past its glass transition temperature (Tg) between 120 and 140 K, LDA is more viscous than normal water. Recent studies have shown the viscous liquid stays in this alternative form of liquid water up to somewhere between 140 and 210 K, a temperature range that is also inhabited by ice Ic. LDA has a density of 0.94 g/cm3, less dense than the densest water (1.00 g/cm3 at 277 K), but denser than ordinary ice (ice Ih).
Hyperquenched glassy water (HGW) is formed by spraying a fine mist of water droplets into a liquid such as propane around 80 K or by hyperquenching fine micrometer-sized droplets on a sample-holder kept at liquid nitrogen temperature, 77 K, in a vacuum. Cooling rates above 104 K/s are required to prevent crystallization of the droplets. At liquid nitrogen temperature, 77 K, HGW is kinetically stable and can be stored for many years.
High Density Amorphous
High-density amorphous ice (HDA) can be formed by compressing ice Ih at temperatures below ~140 K. At 77 K, HDA forms from ice Ih at around 1.6 GPa and from LDA at around 0.5 GPa (approximately 5,000 atm). At this temperature, it can be recovered back to ambient pressure and kept indefinitely. At these conditions (ambient pressure and 77 K), HDA has a density of 1.17 g/cm3.
Peter Jenniskens and David F. Blake demonstrated in 1994 that a form of high-density amorphous ice is also created during vapor deposition of water on low-temperature (< 30 K) surfaces such as interstellar grains. The water molecules do not fully align to create the open cage structure of low-density amorphous ice. Many water molecules end up at interstitial positions. When warmed above 30 K, the structure re-aligns and transforms into the low-density form.
Very High Density Amorphous
Very-high-density amorphous ice (VHDA) was discovered in 1996 by Mishima who observed that HDA became denser if warmed to 160 K at pressures between 1 and 2 GPa and has a density of 1.26 g/cm3 at ambient pressure and temperature of 77 K. More recently it was suggested that this denser amorphous ice was a third amorphous form of water, distinct from HDA, and was named VHDA.
Hexagonal (Ih)
This form of ice is the form all natural ice on Earth conforms to. It has a hexagonal crystal structure and has a low density structure. It has a low packing efficiency compared to other ices, such as cubic. This ice forms in sheets, much like mica. This sheeted structure is characteristic to minerals with basal cleavage. The hardness varies with temperature. At 0 °C, the hardness is about or below 2 on the Mohs scale and at -80 °C it is at 6 on the scale. Crystals of this ice forms hexagonal plates and/or columns. With increasing pressure, thermal conductivity of the ice decreases. This is caused by changes in the bonding of hydrogen that decreases the transverse sound velocity. The nucleation of this ice is enhanced at the air-water surface than within the water (by a factor of 10^10). Crystals grow in the direction of the c-axis. They either grow inside vertical freezing pipes or grow down vertically from platelets already nucleated. They can also grow from prism faces in an agitated environment. The speeds of growth depends on the ability for the crystal faces to form greater amounts of cooperative hydration. The temperature of the surrounding water determines to amount of branching in the crystal. There is more branching at a low degree (<2 °C) and more needle like growth at a higher degree (>4 °C). Solutes in the water cannot be incorporated in a hexagonal structure. The solutes are expelled to the surface or the amorphous layer between microcrystalline crystals.
Cubic (Ic)
Cubic ice is a form of water ice commonly found in high clouds in the Earth's atmosphere. It is a metastable form of ice that can be formed by condensing water vapor at ambient pressure and low temperatures (generally less than -80 degrees Celsius), at -38 degrees Celsius in small droplets, or by reducing the pressure on high pressure ices at 77 K. Cubic ice has a higher vapor pressure than ice Ih. It is often found in freezing confined aqueous systems. It is thought that this ice may be the preferred form for water droplets under 15 nm radius at around 160-220 K. This is due to how cubic ice has lower interfacial free energy than hexagonal ice. Large cubic crystals convert slowly to hexagonal ice at 170-220 K. Cubic ice consists of a face centered cubic lattice. The ice has a fairly open, low density structure. Cubic ice has a staggered arrangement of hydrogen bonding, instead of hexagonal ice's 3/4 arrangement of hydrogen bonding. All molecules have identical environments. All atoms have four tetrahedrally arranged nearest neighbors and twelve second neighbors. The H-O-H angle of the water molecules do not change much from the isolate form of the molecule. The hydrogen bonds are not straight in the ice structure. Cubic ice, much like hexagonal, shows a reduction in thermal conductivity with increasing pressure. This is caused, just like hexagonal, by changes in hydrogen bonding decreasing the transverse sound velocity.
Ice II
Ice II is a rhombohedral crystalline form of ice with highly ordered structure. It is formed from ice Ih by compressing it at temperature of 198 K at 300 MPa or by decompressing ice V at 238K. When heated it undergoes transformation to ice III, but it is not easily formed by cooling ice III. It is thought that the cores of icy moons like Jupiter's Ganymede may be made of ice II. In ice II, all water molecules are hydrogen bonded to four others, two as donor and two as acceptor. Ice-two may exist metastably below ~100 K between ambient pressure and ~5 GPa. At ambient pressure it irreversibly transforms into ice Ic above 160 K. As the H-O-H angle does not vary much from that of the isolated molecule, the hydrogen bonds are not straight. Half the open hexagonal channels of ice Ih have collapsed in ice II. The relationship of the ice II structure to ice Ih can be visualized by detaching the columns of hexameric ice Ih rings, moving them relatively up or down at right angles to their plane, rotating them about 30° around this axis and re-linking the hydrogen bonds in a more compact way to give a density of 1.16 g/cm3. The hydrogen bonding is ordered and fixed in ice II. There is no corresponding disordered phase, in contrast to the other ordered ices VIII, IX, XI and XV. The lack of a disordered phase has been correlated with the high energy difference between the most and the second most stable ice configurations. Some of ice II's hydrogen bonds are bent and, consequentially, much weaker than the hydrogen bonds in hexagonal ice.[1]
Ice III
Ice III is a type of ice that is formed from water at 300 MPa with a temperature lowered to 250 K. The unit cell forms tetragonal crystals with a space group of P41212 92, a Laue class symmetry of 4/mmm, and analogous to keatite silica. All the water molecules are hydrogen bonded to four others and the ICE III contains five membered rings joined as bicylo-heptamers with a density of 1.16 g cm-3. The disorder hydrogen bonding constantly changes with the tetragonal crystal being pseudo-cubic with cell dimensions 6.666 angstroms and 6.936 angstroms
Ice IV
Ice IV is a type of water formed by the heating of high-density amorphous ice slowly (0.4 K min-1) at 145 K and at a constant pressure of 0.81 GPa.
Sites of Possible Extraterrestrial Life
This section is incomplete. |
For the 2014-15 Solar event, it is useful to know which celestial bodies in our solar system could possibly have life living on (or in) them.
Mars
Life needs many things to survive. It needs water, which Mars has in the form of ice at its poles. If life ever evolved on Mars, it did so in a place with a source of liquid water. Ice caps at the poles will not sustain life, but other sources of water might work, such as hydrothermal pools, not unlike those at Yellowstone National Park in Wyoming, or any other place that will have the temperature to sustain liquid water.
Life also needs energy to thrive. The presence of energy on Mars other than sunlight is rare, since there are superoxides that break down organic matter that life is based on. Besides sunlight, there is little evidence of other energy, such as chemical or geothermal energy like Earth, but it may still be there. On Earth, life can be sustained in many places where the sunlight never reaches, such as abysses, trenches, and caves with chemical energy, or in the Earth's crust with geothermal energy. There is a chance that the same phenomenons can happen on Mars like they did on Earth.
NASA (National Aeronautics and Space Administration[2]) is looking for signs of life on Mars. They are looking for signs that will allude to the conditions on Mars that may have allowed life to be sustained there, such as carbon. The element carbon (C) is the building block of life. All living things use carbon. Dead organisms turn into peat, which turns into coal, which has a high carbon percentage, and then we humans burn the coal to produce carbon energy. It is known that the atmosphere of Mars is carbon dioxide (CO2). If NASA finds carbonate minerals on Mars, we will know that there was once liquid water on Mars because the reaction between water (H2O) and the atmosphere (CO2) will react to form carbonic acid, or H2CO3, hydrogen carbonate. If these are found, then we will know that liquid water has existed on Mars for a long time, maybe even enough for life to have developed. On Earth, fossils can be used to tell our geologic and biologic history. Maybe Mars will have fossils, too. NASA is currently looking into dried lake/riverbeds for possible evidence of life in fossils.
Europa
The moon of Europa is one of the top locations in the solar system for the potential of extraterrestrial life. Life has three main requirements for survival, the presence of natural elements and chemicals, the presence of a universal solvent, and an ample supply of energy. Europa has the potential of all of these three. The theorized water ocean under the moon's surface is the perfect solvent for natural chemicals. Ridges on Europa's surface have a reddish color created by certain natural elements. This may be an indication of the presence of these chemicals in the underground ocean. Tidal heating from Jupiter, radioactive decay, and hydrothermal vents at the ocean floor all are supposed energy sources for life on the moon. Because all three requirements are present in the supposed ocean, if life is on Europa, it would most likely be located in the ocean. Where they are located in the ocean could vary. Life here would be extremophiles living either near hydrothermal vents on the ocean floor, on the underside of the icy surface, freely floating in the ocean, or even within the rock of the ocean floor (like endoliths on Earth) Life could also be found in lakes encased by the ice layer, separate from the ocean. Life on the moon would be single cellular or small multi cellular extremophiles. If the ocean environment was extremely salty, only extreme halophiles would be able to thrive. If life isn't on Europa now, it could appear later due to changes in the composition of the ocean salinity.
Enceladus
Saturn's moon Enceladus is another possible source of extraterrestrial life. Since the discovery in 2005 that Enceladus actively vents gas, the spacecraft Cassini has provided NASA with more information that will help them determine if life can be sustained on this remote moon.
Many flybys and analyses by Cassini of the plumes of water that stream through Enceladus have revealed that there are chemicals like carbon, hydrogen, nitrogen, and oxygen present underneath the ice shell of Enceladus' crust, which is a giant ocean. This information was only further confirmed when, in 2015, Cassini flew into a plume of water and used its chemical detector to confirm that there is molecular hydrogen (H2) on Enceladus. On Earth, molecular hydrogen sprouts from the hydrothermal vents on the ocean floor, entering the ocean. The chemistry between elements in the water and the molecular hydrogen supports life forms such as one-celled microbes, tube worms, and crabs. If this can occur on Earth, then there is a very high chance of it occurring on Enceladus. Three things are required for life to be sustained, and Enceladus has the potential for all of them: chemicals (existing), water (from the acidic water under the 5km shell of ice), and energy (from the plumes of water).