Page 1 of 3
Gas Expansion Task
Posted: September 5th, 2017, 9:25 am
by Unome
See rule 4.b.xi for details.
The specifics of the rules seem to prevent CO2 production from carbonic acid or similar, since the expansion has to be from a thermal reaction.
Re: Gas Expansion Task
Posted: September 30th, 2017, 3:50 pm
by raxu
Does a thermal reaction mean that we have to change temperature in
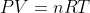
? If we can change pressure, I was thinking about activating it with a pulley task where a piston is lifted.
Re: Gas Expansion Task
Posted: September 30th, 2017, 6:38 pm
by andrew lorino
raxu wrote:Does a thermal reaction mean that we have to change temperature in
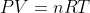
? If we can change pressure, I was thinking about activating it with a pulley task where a piston is lifted.
I thought that it meant that the heat had to expand the piston, not vice versa.
Re: Gas Expansion Task
Posted: October 1st, 2017, 7:36 am
by ScottMaurer19
andrew lorino wrote:raxu wrote:Does a thermal reaction mean that we have to change temperature in
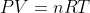
? If we can change pressure, I was thinking about activating it with a pulley task where a piston is lifted.
I thought that it meant that the heat had to expand the piston, not vice versa.
I thought the same as Andrew. The gas must gain thermal energy, and, as a result, expand.
Re: Gas Expansion Task
Posted: October 16th, 2017, 9:55 am
by ScottMaurer19
I wonder if lighting a match in a sealed container would count.
Re: Gas Expansion Task
Posted: October 16th, 2017, 11:01 am
by andrew lorino
ScottMaurer19 wrote:I wonder if lighting a match in a sealed container would count.
Maybe, the amount of gas wouldn't change (in theory), so the expansion would just be thermal, but proving that to an ES would be difficult.
Re: Gas Expansion Task
Posted: October 16th, 2017, 3:50 pm
by chalker
andrew lorino wrote:ScottMaurer19 wrote:I wonder if lighting a match in a sealed container would count.
Maybe, the amount of gas wouldn't change (in theory), so the expansion would just be thermal, but proving that to an ES would be difficult.
Your theory is incorrect. Check out this video for an example of a classic science experiment:
https://www.youtube.com/watch?v=pwNFnOx5iEg
Re: Gas Expansion Task
Posted: October 16th, 2017, 5:49 pm
by andrew lorino
chalker wrote:andrew lorino wrote:ScottMaurer19 wrote:I wonder if lighting a match in a sealed container would count.
Maybe, the amount of gas wouldn't change (in theory), so the expansion would just be thermal, but proving that to an ES would be difficult.
Your theory is incorrect. Check out this video for an example of a classic science experiment:
https://www.youtube.com/watch?v=pwNFnOx5iEg
The result is not because of a change in the amount of gas. This can be shown with the equation: 1mol O2 + 1mol C --> 1mol CO2 (Of course this doesn't factor in the other stuff in a match). Because the match is lit before the glass is placed atop it, the pressure remains equalized with the atmosphere whilst the temperature remains higher than datum. Once the match flame dies, the temperature decreases, and, according to Charles' law, the volume decreases. If the flame were lit in a sealed environment, the pressure should increase.
Re: Gas Expansion Task
Posted: October 17th, 2017, 4:36 am
by ScottMaurer19
chalker wrote:andrew lorino wrote:ScottMaurer19 wrote:I wonder if lighting a match in a sealed container would count.
Maybe, the amount of gas wouldn't change (in theory), so the expansion would just be thermal, but proving that to an ES would be difficult.
Your theory is incorrect. Check out this video for an example of a classic science experiment:
https://www.youtube.com/watch?v=pwNFnOx5iEg
Well if the volume decreases than I might have a hard time getting the gas to expand anyway (other than the initial burn).
Re: Gas Expansion Task
Posted: October 17th, 2017, 3:32 pm
by chalker
andrew lorino wrote:chalker wrote:andrew lorino wrote:
Maybe, the amount of gas wouldn't change (in theory), so the expansion would just be thermal, but proving that to an ES would be difficult.
Your theory is incorrect. Check out this video for an example of a classic science experiment:
https://www.youtube.com/watch?v=pwNFnOx5iEg
The result is not because of a change in the amount of gas. This can be shown with the equation: 1mol O2 + 1mol C --> 1mol CO2 (Of course this doesn't factor in the other stuff in a match). Because the match is lit before the glass is placed atop it, the pressure remains equalized with the atmosphere whilst the temperature remains higher than datum. Once the match flame dies, the temperature decreases, and, according to Charles' law, the volume decreases. If the flame were lit in a sealed environment, the pressure should increase.
You're right. Thanks for correcting me on what was an incorrect assumption.