When a student mixes 50 mL of 1.0 M HCl and 50 mL of 1.0 M NaOH in a coffee-cup calorimeter, the temperature of the resultant solution increases from 21.0 °C to 27.5 °C. Calculate the enthalpy change for the reaction in kJ per mol of HCl, assuming that the calorimeter loses only a negligible quantity of heat. The total volume of the solution is 100 mL, its density is 1.0 g/mL, and its specific heat is 4.18 J/g-K.
Thermodynamics B/C
- TheChiScientist
- Member

- Posts: 732
- Joined: March 11th, 2018, 11:25 am
- Division: Grad
- State: IL
- Pronouns: He/Him/His
- Has thanked: 6 times
- Been thanked: 44 times
Re: Thermodynamics B/C
Straight outta AP Chem.  (Question is aimed at Div. C. Sorry Div. B!)
(Question is aimed at Div. C. Sorry Div. B!)
When a student mixes 50 mL of 1.0 M HCl and 50 mL of 1.0 M NaOH in a coffee-cup calorimeter, the temperature of the resultant solution increases from 21.0 °C to 27.5 °C. Calculate the enthalpy change for the reaction in kJ per mol of HCl, assuming that the calorimeter loses only a negligible quantity of heat. The total volume of the solution is 100 mL, its density is 1.0 g/mL, and its specific heat is 4.18 J/g-K.
When a student mixes 50 mL of 1.0 M HCl and 50 mL of 1.0 M NaOH in a coffee-cup calorimeter, the temperature of the resultant solution increases from 21.0 °C to 27.5 °C. Calculate the enthalpy change for the reaction in kJ per mol of HCl, assuming that the calorimeter loses only a negligible quantity of heat. The total volume of the solution is 100 mL, its density is 1.0 g/mL, and its specific heat is 4.18 J/g-K.
A Science Olympian from 2015 - 2019 CLCSO Alumni
Medal Count:30
IL PPP/Mission Assistant State Supervisor.
CLC Div. B Tournament Director.
President of The Builder Cult.
"A true Science Olympian embraces a life without Science Olympiad by becoming a part of Science Olympiad itself"- Me
Medal Count:30
IL PPP/Mission Assistant State Supervisor.
CLC Div. B Tournament Director.
President of The Builder Cult.
"A true Science Olympian embraces a life without Science Olympiad by becoming a part of Science Olympiad itself"- Me
-
UTF-8 U+6211 U+662F
- Exalted Member

- Posts: 1597
- Joined: January 18th, 2015, 7:42 am
- Division: C
- State: PA
- Has thanked: 6 times
- Been thanked: 15 times
Re: Thermodynamics B/C
TheChiScientist wrote:Straight outta AP Chem.(Question is aimed at Div. C. Sorry Div. B!)
When a student mixes 50 mL of 1.0 M HCl and 50 mL of 1.0 M NaOH in a coffee-cup calorimeter, the temperature of the resultant solution increases from 21.0 °C to 27.5 °C. Calculate the enthalpy change for the reaction in kJ per mol of HCl, assuming that the calorimeter loses only a negligible quantity of heat. The total volume of the solution is 100 mL, its density is 1.0 g/mL, and its specific heat is 4.18 J/g-K.
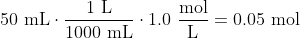
(Since we're considering a range, actually converting the temperatures to Kelvin would yield the same answer.)
Technically, the final answer should have one significant figure, but...
Last edited by UTF-8 U+6211 U+662F on October 18th, 2018, 5:28 pm, edited 1 time in total.
- TheChiScientist
- Member

- Posts: 732
- Joined: March 11th, 2018, 11:25 am
- Division: Grad
- State: IL
- Pronouns: He/Him/His
- Has thanked: 6 times
- Been thanked: 44 times
Re: Thermodynamics B/C
Nice. AP Chem HW done!UTF-8 U+6211 U+662F wrote:TheChiScientist wrote:Straight outta AP Chem.(Question is aimed at Div. C. Sorry Div. B!)
When a student mixes 50 mL of 1.0 M HCl and 50 mL of 1.0 M NaOH in a coffee-cup calorimeter, the temperature of the resultant solution increases from 21.0 °C to 27.5 °C. Calculate the enthalpy change for the reaction in kJ per mol of HCl, assuming that the calorimeter loses only a negligible quantity of heat. The total volume of the solution is 100 mL, its density is 1.0 g/mL, and its specific heat is 4.18 J/g-K.Side note: I wish the built-in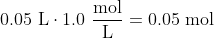
(Since we're considering a range, actually converting the temperatures to Kelvin would yield the same answer.)
Technically, the final answer should have one significant figure, but...
renderer included the SI package...
Your turn.
A Science Olympian from 2015 - 2019 CLCSO Alumni
Medal Count:30
IL PPP/Mission Assistant State Supervisor.
CLC Div. B Tournament Director.
President of The Builder Cult.
"A true Science Olympian embraces a life without Science Olympiad by becoming a part of Science Olympiad itself"- Me
Medal Count:30
IL PPP/Mission Assistant State Supervisor.
CLC Div. B Tournament Director.
President of The Builder Cult.
"A true Science Olympian embraces a life without Science Olympiad by becoming a part of Science Olympiad itself"- Me
-
UTF-8 U+6211 U+662F
- Exalted Member

- Posts: 1597
- Joined: January 18th, 2015, 7:42 am
- Division: C
- State: PA
- Has thanked: 6 times
- Been thanked: 15 times
Re: Thermodynamics B/C
A gas is allowed to freely expand to a box which is five times the volume the previous container was. What is the resulting entropy change? A gas expands reversibly until its volume is five times as much. What is the resulting entropy change? Explain the difference between free expansion and reversible expansion.
-
MattChina
- Member

- Posts: 225
- Joined: February 12th, 2017, 8:06 am
- Division: B
- State: NY
- Has thanked: 0
- Been thanked: 0
Re: Thermodynamics B/C
5 times, 0, free expansion is irreversible and the entropy change is greater than zero, while reversible expansion has no entropy change.UTF-8 U+6211 U+662F wrote:A gas is allowed to freely expand to a box which is five times the volume the previous container was. What is the resulting entropy change? A gas expands reversibly until its volume is five times as much. What is the resulting entropy change? Explain the difference between free expansion and reversible expansion.
2019 events: Water Quality, Battery Buggy, Elastic Launch Glider, Density Lab, Circuit Lab, Thermodynamics
R.C Murphy Co-Captain
Dank Memes Area Homeschool Team member
R.C Murphy Co-Captain
Dank Memes Area Homeschool Team member
-
UTF-8 U+6211 U+662F
- Exalted Member

- Posts: 1597
- Joined: January 18th, 2015, 7:42 am
- Division: C
- State: PA
- Has thanked: 6 times
- Been thanked: 15 times
Re: Thermodynamics B/C
MattChina wrote:5 times, 0, free expansion is irreversible and the entropy change is greater than zero, while reversible expansion has no entropy change.UTF-8 U+6211 U+662F wrote:A gas is allowed to freely expand to a box which is five times the volume the previous container was. What is the resulting entropy change? A gas expands reversibly until its volume is five times as much. What is the resulting entropy change? Explain the difference between free expansion and reversible expansion.
1) Increases by [math]Nk_B\ln 5[/math] or equivalently [math]nR\ln 5[/math], where N is the number of particles, k is the Boltzmann constant, n is the number of moles, and R is the ideal gas constant 2) Yep! Just note that only the net entropy change (of both the gas and the surroundings) is 0. The entropy of the gas itself can increase or decrease. 3) Yep!
-
UTF-8 U+6211 U+662F
- Exalted Member

- Posts: 1597
- Joined: January 18th, 2015, 7:42 am
- Division: C
- State: PA
- Has thanked: 6 times
- Been thanked: 15 times
Re: Thermodynamics B/C
What were Clausius's and Kelvin's formulations of the Second Law of Thermodynamics?
- Crimesolver
- Member

- Posts: 111
- Joined: July 31st, 2018, 2:07 pm
- Division: C
- State: CA
- Pronouns: She/Her/Hers
- Has thanked: 6 times
- Been thanked: 22 times
Re: Thermodynamics B/C
Clausius's formulation was "it is impossible for a self-acting, unaided by any external agency, to trader heat from a body at lower temperature to a body, at a high temperature.UTF-8 U+6211 U+662F wrote:What were Clausius's and Kelvin's formulations of the Second Law of Thermodynamics?
Kelvin's formulation was "it is impossible to obtain a continuous supply of energy by cooling a body below the coldest of it's surroundings."
-
UTF-8 U+6211 U+662F
- Exalted Member

- Posts: 1597
- Joined: January 18th, 2015, 7:42 am
- Division: C
- State: PA
- Has thanked: 6 times
- Been thanked: 15 times
Re: Thermodynamics B/C
Yep, your turn.Crimesolver wrote:Clausius's formulation was "it is impossible for a self-acting, unaided by any external agency, to trader heat from a body at lower temperature to a body, at a high temperature.UTF-8 U+6211 U+662F wrote:What were Clausius's and Kelvin's formulations of the Second Law of Thermodynamics?
Kelvin's formulation was "it is impossible to obtain a continuous supply of energy by cooling a body below the coldest of it's surroundings."
Who is online
Users browsing this forum: No registered users and 0 guests

