Re: Materials Science C
Posted: April 8th, 2018, 8:21 pm
To speed things up can we post and answer simultaneously and if the answer is incorrect, correct them later? Because this thread is going kinda slowly
Works for me, I definitely want this to move fasterName wrote:To speed things up can we post and answer simultaneously and if the answer is incorrect, correct them later? Because this thread is going kinda slowly
Ok, name this chemical (I think it could be covered in materials science):IcsTam wrote:So for filler, I would specify that it improves tensile strength and directional stability in the polymer. Otherwise, that's right! Your turnJavaScriptCoder wrote:Well, this forum seems a little dead, so I'll revitalize it.
A plasticizer makes a material less brittle, a filler (name is a bit self-explanatory) fills gaps, a stabilizer prevents the breakdown of an emulsion (from what I've learned liquid-liquid colloids are insanely unstable) and a lubricant decreases friction.IcsTam wrote:Describe the function of each of the following: Plasticizer, Filler, Stabilizer, Lubricant.
Is it right?

Benzoyl CyanideJavaScriptCoder wrote:Ok, name this chemical (I think it could be covered in materials science):IcsTam wrote:So for filler, I would specify that it improves tensile strength and directional stability in the polymer. Otherwise, that's right! Your turnJavaScriptCoder wrote:Well, this forum seems a little dead, so I'll revitalize it.
A plasticizer makes a material less brittle, a filler (name is a bit self-explanatory) fills gaps, a stabilizer prevents the breakdown of an emulsion (from what I've learned liquid-liquid colloids are insanely unstable) and a lubricant decreases friction.
Is it right?
Very well, I'll give that to you. The IUPAC name is technically phenylglyoxylnitrile but it is more commonly known as that. Your turn (make sure to include your question in the answer so it goes faster).IcsTam wrote:Benzoyl CyanideJavaScriptCoder wrote:Ok, name this chemical (I think it could be covered in materials science):IcsTam wrote:
So for filler, I would specify that it improves tensile strength and directional stability in the polymer. Otherwise, that's right! Your turn
Free-radicals are particles that have unpaired valence electrons. The process of free radical polymerization is like chain-growth polymerization: Free radicals append themselves to the end of the polymer chain.IcsTam wrote:Explain the process of free-radical polymerization.
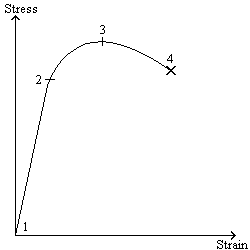 .
. 1 is the material at rest (is there a more precise term for this?). 2 is the yield point. 3 is the ultimate tensile strength. 4 is fractureJavaScriptCoder wrote:Free-radicals are particles that have unpaired valence electrons. The process of free radical polymerization is like chain-growth polymerization: Free radicals append themselves to the end of the polymer chain.IcsTam wrote:Explain the process of free-radical polymerization.
Next question.
.
Name the points on the stress-strain curve (1, 2, 3, 4).
BONUS POINTS:

Which of these curves is most likely to be representative of a thermoplastic polymer? (Getting the images without labels was the hardest part tbh)