Page 2 of 23
Re: Thermodynamics B/C
Posted: September 9th, 2017, 8:55 am
by Raleway
WhatScience? wrote:Explain the difference in between entropy driven and enthalpy driven reactions.
When using Gibb's Free Energy Equation (dG = dH - TdS) or any derivative form of that with Enthalpy and Entropy in it, that the larger of the two expressions (entropy or enthalpy) that make the reaction spontaneous will be said to have "driven" the reaction.
Question: When reading a phase change diagram. describe the kinetic energy at the triple point relative to the other three states' kinetic energy.
Re: Thermodynamics B/C
Posted: September 9th, 2017, 10:32 am
by WhatScience?
Raleway wrote:
Question: When reading a phase change diagram. describe the kinetic energy at the triple point relative to the other three states' kinetic energy.
This is what I have in relation to a phase change diagram of water. Statements would be different for something like the diagram of carbon dioxide...
The triple point is at the highest average kinetic energy possible for a solid.
A gas' average kinetic energy will be higher than the triple point when the amount of pressure is above the triple point, but lower than the triple point when the pressure is lower than the triple point.
Since at most pressure levels, liquid has a freezing point and a boiling point, the average kinetic energy of a liquid can at any given pressure be both higher or lower than the average kinetic energy at the triple point.
Re: Thermodynamics B/C
Posted: September 9th, 2017, 10:46 am
by WhatScience?
Question: van der waals equation is a correction of the ideal gas law. In which three scenarios is it most applicable and what is the reasoning behind the changes made to the equation?
Re: Thermodynamics B/C
Posted: September 10th, 2017, 10:01 am
by Kavar
wzhang5460 wrote:D
Credits to Khan Academy lol.
I believe you are mistaken. The answer should be C if you cancel out the values correctly. Credits to Khan Academy


.
Re: Thermodynamics B/C
Posted: September 10th, 2017, 12:22 pm
by WhatScience?
WhatScience? wrote:Question: van der waals equation is a correction of the ideal gas law. In which three scenarios is it most applicable and what is the reasoning behind the changes made to the equation?
Re: Thermodynamics B/C
Posted: September 10th, 2017, 12:22 pm
by WhatScience?
answer the above question please
Re: Thermodynamics B/C
Posted: September 11th, 2017, 1:58 pm
by wzhang5460
Kavar wrote:wzhang5460 wrote:D
Credits to Khan Academy lol.
I believe you are mistaken. The answer should be C if you cancel out the values correctly. Credits to Khan Academy


.
Sorry, I typed the wrong letter. Haha
Re: Thermodynamics B/C
Posted: September 12th, 2017, 3:37 pm
by WhatScience?
WhatScience? wrote:WhatScience? wrote:Question: van der waals equation is a correction of the ideal gas law. In which three scenarios is it most applicable and what is the reasoning behind the changes made to the equation?
Re: Thermodynamics B/C
Posted: October 6th, 2017, 3:36 pm
by raxu
I only know two scenarios: when the molecules are relatively large (e.g. octane) and when there is strong intermolecular interactions (e.g. water vapor).
The equation is
(V-nb)=nRT)
. The correction factor

accounts for intermolecular interactions, and the correction factor
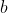
accounts for size of gases.
The bob temperature scale is a linear temperature scale where at 1 atm, water freezes at 42°B and boils at 239°B. Convert -53 °C to °B.
Re: Thermodynamics B/C
Posted: October 9th, 2017, 2:42 pm
by WhatScience?
raxu wrote:I only know two scenarios: when the molecules are relatively large (e.g. octane) and when there is strong intermolecular interactions (e.g. water vapor).
The equation is
(V-nb)=nRT)
. The correction factor

accounts for intermolecular interactions, and the correction factor
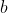
accounts for size of gases.
The bob temperature scale is a linear temperature scale where at 1 atm, water freezes at 42°B and boils at 239°B. Convert -53 °C to °B.
The equation part is right but the three scenarios I was looking for was any scenario where the molecules are crowded together:
High pressure, Low Temperature, and High Density
Either way, your turn.